Our Focus
“The MC4R pathway plays a pivotal role in the underlying biology of many forms of severe obesity and hyperphagia. We are committed to using our growing understanding of this pathway to develop new therapeutic options that can help to relieve the substantial burden of living with these rare diseases.”
– Dana Washburn, Senior Vice President, Clinical Development
Setmelanotide, our melanocortin-4 receptor (MC4R) pathway agonist, has the potential to restore the function of an impaired MC4R pathway, and in doing so, help to reduce hunger and body weight in individuals living with severe obesity and hyperphagia.
We are focused on developing setmelanotide as a potential therapy to treat rare MC4R pathway diseases. Clinically, these diseases share the hallmark characteristics of early-onset, severe obesity and insatiable hunger or hyperphagia. In addition to severe obesity, usually beginning before the age of 6 and intense food-seeking behavior from hyperphagia, patients with these diseases often have additional co-morbidities that are associated with obesity.
Rhythm is focused on investigating the efficacy and safety of setmelanotide in the treatment of several diseases, which may be caused by variants in one of many genes related to the MC4R pathway.
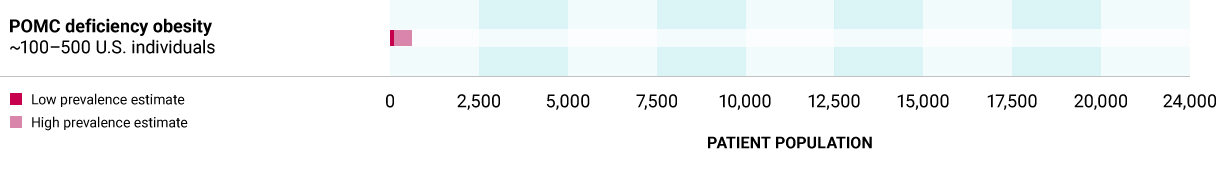
Proopiomelanocortin (POMC) deficiency obesity
Caused by biallelic variants in either the POMC gene, the source of melancyte-stimulating hormone (MSH), or Proprotein convertase Subtilisin/Kexin Type 1 (PCSK1) gene, which is required for producing MSH from POMC, leading to an absence or decrease in MSH. POMC deficiency is characterized by hyperphagia, rapid weight gain leading to severe obesity, often in early infancy, with individuals demonstrating remarkable weight increases.
~100 – 500 U.S. individuals
Alström syndrome
Caused by variants in the ALMS1 gene, Alström syndrome is a rare genetic disorder of obesity associated with impaired cilia function. Cilia dysfunction in the hypothalamus can lead to MC4R pathway dysfunction.
Alström syndrome can lead to childhood obesity and hyperphagia as well as progressive vision loss, hearing loss, cardiomyopathy, and other clinical symptoms.
~500 – 1,000 worldwide individuals

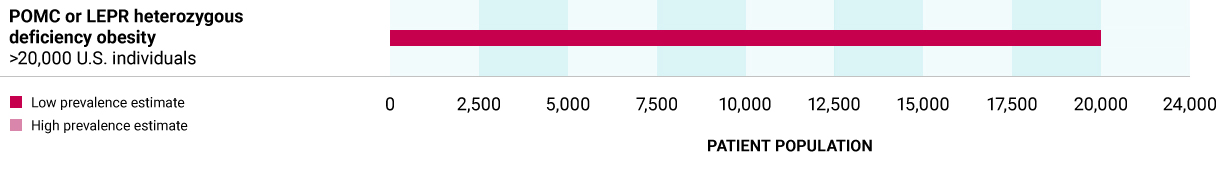
POMC or LEPR heterozygous deficiency obesity (HETs)
Caused by high-impact heterozygous variants in one of the POMC, PCSK1 or LEPR genes, that may lead to decreased production of MSH and predisposition to obesity.
>20,000 U.S. individuals
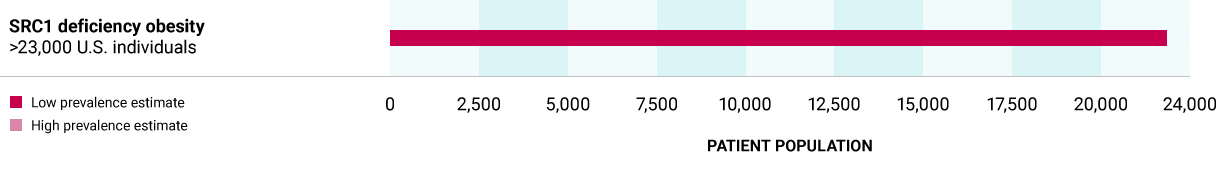
SRC1 deficiency obesity
Caused by heterozygous variants in the SRC1 gene, a transcriptional coactivator protein, leading to decreased POMC expression in POMC-producing neurons.
>23,000 U.S. individuals
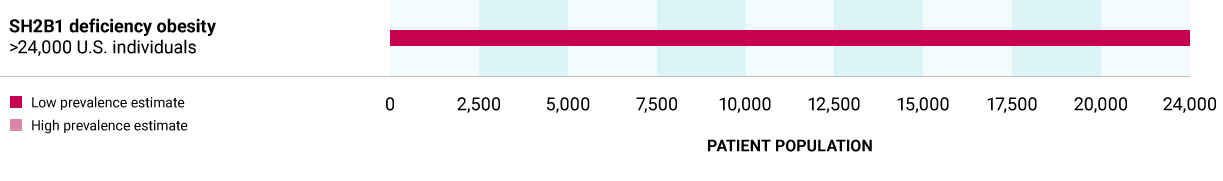
SH2B1 deficiency obesity
Caused by heterozygous variants in the SH2B1 gene, which codes for the adaptor protein, leading to decreased LEPR activation. Deficiency in SH2B1 can arise through a variant in the SH2B1 gene or through chromosomal deletions (chromosome 16) that encompass the SH2B1 gene. In both cases, there is a dysfunction/loss of only one copy of the SH2B1 gene.
>24,000 U.S. individuals
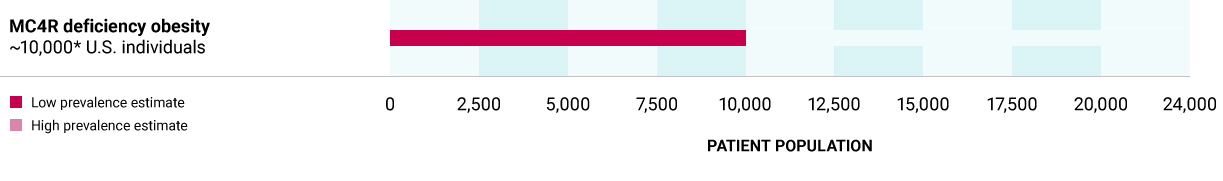
MC4R deficiency obesity
Caused by heterozygous variants in the MC4R gene, which encodes for the MC4R receptor for MSH, leading to insufficient activation of the MC4R pathway. Based on a comprehensive ongoing biochemical screening study, there may be a defined subset of individuals who carry MC4R loss of function variants that may be rescued by an MC4R agonist.
~10,000** U.S. individuals
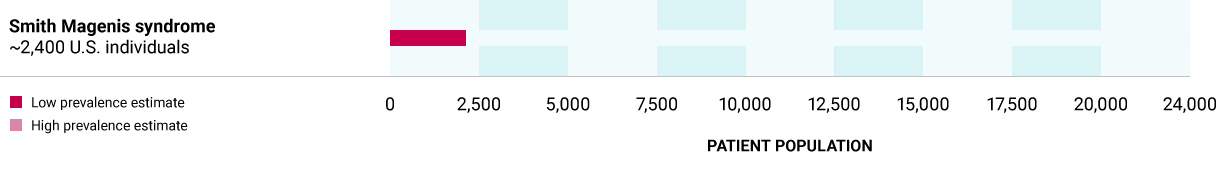
Smith-Magenis syndrome
Caused by heterozygous variants in the RAI1 gene, encoding for transcription factor that affects expression of several MC4R pathway genes. Deficiency in RAI1 can arise through a variant in the RAI1 gene or through chromosomal deletions (chromosome 17) that encompass the RAI1 gene. Hyperphagia and obesity associated with Smith-Magenis syndrome may be caused by an overall decrease in the activation of the MC4R pathway.
~2,400 U.S. individuals
~5M*
People in the United States have early-onset, severe obesityOur sequencing-based epidemiology estimates show that individual genetic diseases of obesity based on a specific variant in one of 36 genes are considered rare or ultra-rare. In the aggregate, however, sequencing data and response rates to setmelanotide in clinical trials show more than 53,000 individuals in the United States with one of these genetic deficiencies have the potential to respond to setmelanotide.
Estimated U.S. prevalence of these diseases is 600 to 2,500 individuals.
We have completed two Phase 3 trials evaluating setmelanotide in these diseases. Obesity due to POMC or LEPR deficiency are ultra-rare diseases caused by variants in POMC, PCSK1 or LEPR genes that impair the MC4 receptor pathway, which is a pathway in the hypothalamus that is responsible for regulating hunger, energy expenditure and consequently body weight. People living with obesity due to POMC or LEPR deficiency struggle with extreme, insatiable hunger beginning at a young age, resulting in early-onset, severe obesity. For more information, visit IMCIVREE.com
Estimated U.S. prevalence of BBS is 4,000 to 5,000 individuals.
BBS is an ultra-rare genetic disease that affects multiple organ systems. Clinical features of BBS may include cognitive impairment, polydactyly, renal dysfunction, hypogonadism, visual impairment, and obesity. Insatiable hunger, also known as hyperphagia, and severe obesity beginning early in life may be common in people living with BBS. Hyperphagia often involves extreme food seeking behavior. We have completed a Phase 3 trial evaluating setmelanotide in patients with BBS. For more information, visit IMCIVREE.com.
We also are studying the association between variants in additional genes with strong or very strong relevance to the MC4R pathway. Our translational research and development (TRAD) team identified these genes based on a validated framework. For more information, see details about our Phase 2 DAYBREAK Trial.
Hypothalamic obesity (HO) is a rare, acquired form of severe obesity that occurs following structural damage to the hypothalamic regions of the brain, most commonly due to a brain tumor or its treatment. This area contains MC4R which is responsible for controlling physiological functions such as hunger and weight regulation. We are evaluating setmelanotide for HO in a Phase 3 study.
Internal Company estimated prevalence of U.S. patients based on company estimates.
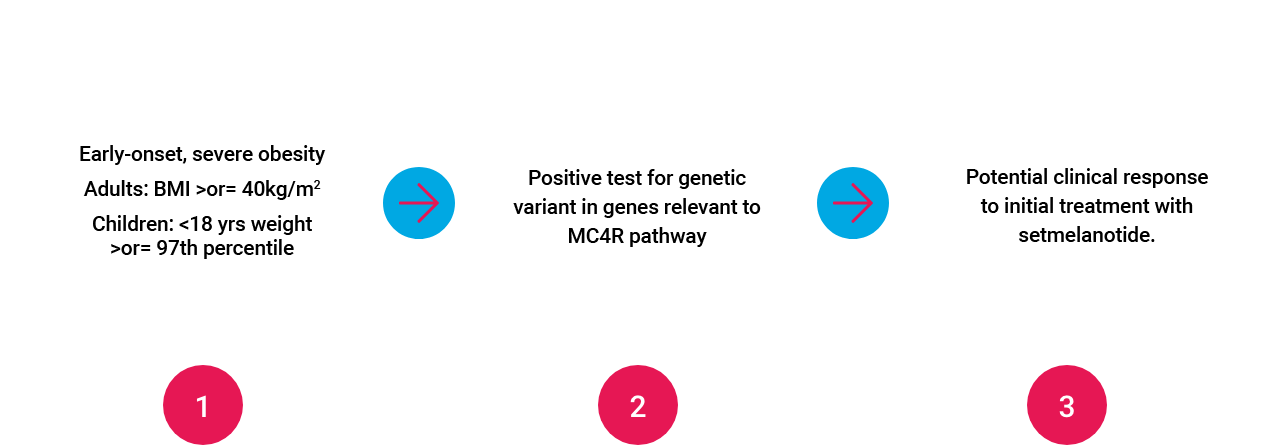
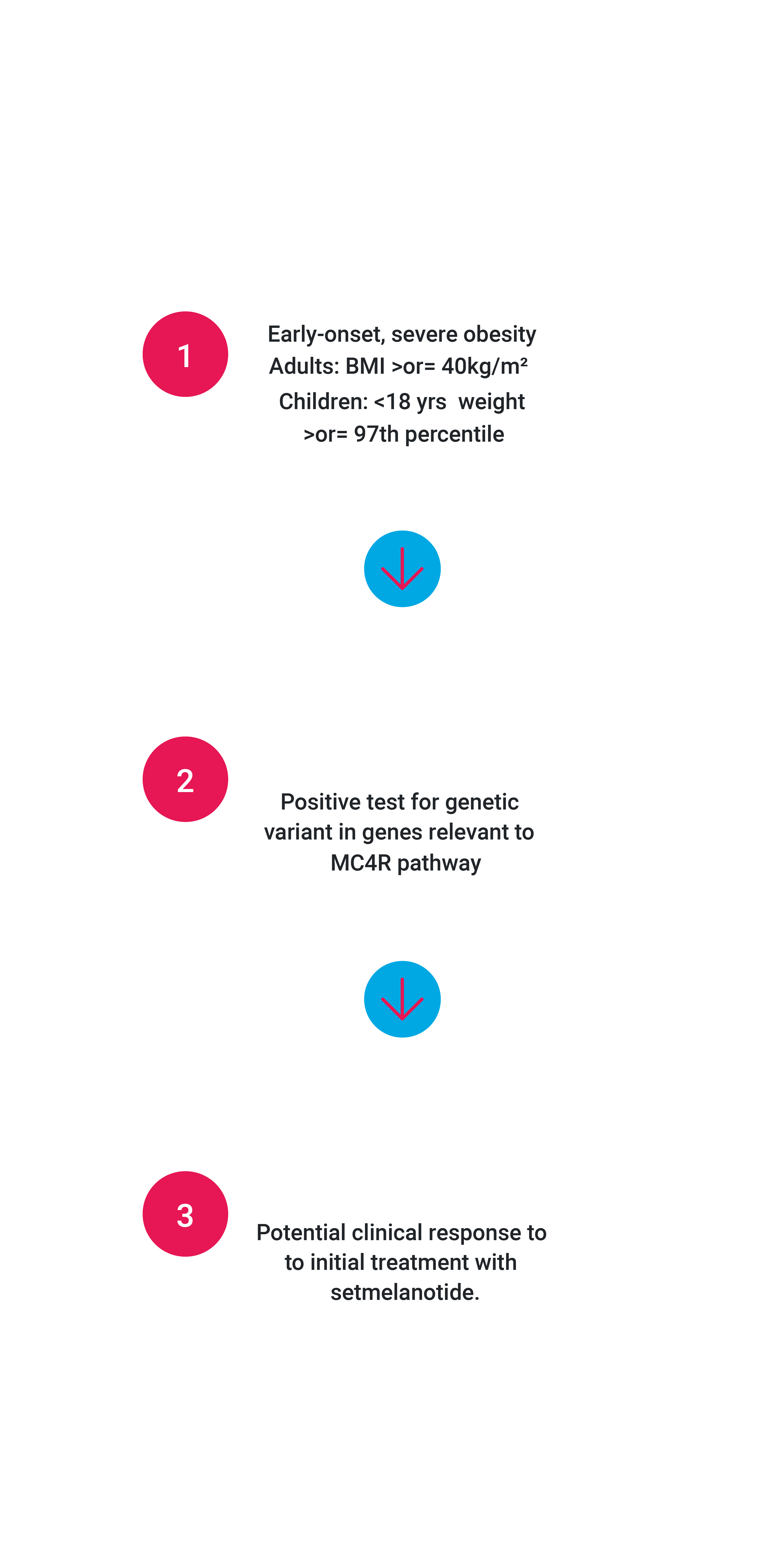
Targeted, 3-step Approach to Identifying and Treating Patients with Rare Genetic Diseases of Obesity
Investigator-initiated Studies
Rhythm’s areas of interest for investigator-initiated studies are focused on MC4R pathway related diseases, including but not limited to natural history, genotype-phenotype correlations, energy expenditure, hyperphagia and real-world experience with setmelanotide. Studies designed for general obesity or non-rare diseases are not within our scope. For more information, please email [email protected].
* 1.7% of the US population (328M; 2019 US census) presents with severe early onset obesity (Hales et al 2018Ɨ); ~95% of individuals with severe early onset obesity remain obese into adulthood (Ward et al 2017);
** Estimated prevalence of U.S. patients based on company estimates;
† Estimated U.S. patients based on population* with early-onset, severe obesity who may benefit from setmelanotide based on sequencing results and current estimated responder rates; does not include ex-U.S. prevalence estimates.